Sensome - SLICE Worldwide 2022
InnovationInnovation: The Clotid (Sensome) – an intelligent guidewire system for a better understanding of the clot
Introduction:
Achieving complete reperfusion is the most important modifiable predictor for maximizing the effect of mechanical thrombectomy treatment resulting in favorable patient functional outcome.
The first-pass effect, defined as a complete or near-complete recanalization after one pass (first-pass effect) of a mechanical thrombectomy device, has been related to better clinical outcome than good recanalization after more than one pass in acute ischemic stroke.
Recent studies have also confirmed that clot composition has a significant bearing on patient outcomes. Red blood cell-rich thrombi, for example, are more susceptible to distal embolisation, while fibrin-rich and platelet-rich thrombi may be harder to remove but also have less chance of embolising distally.
Innovation:
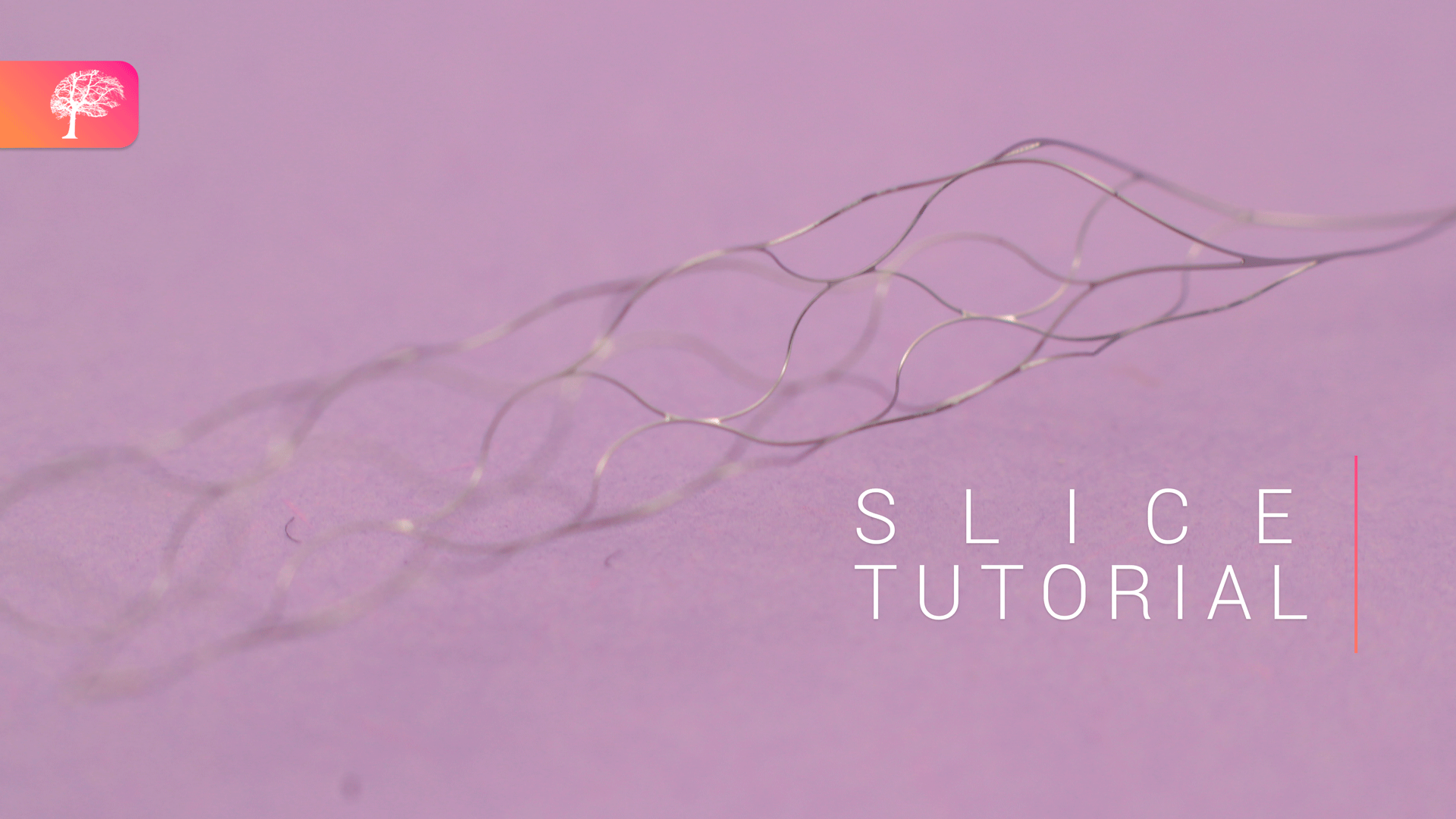
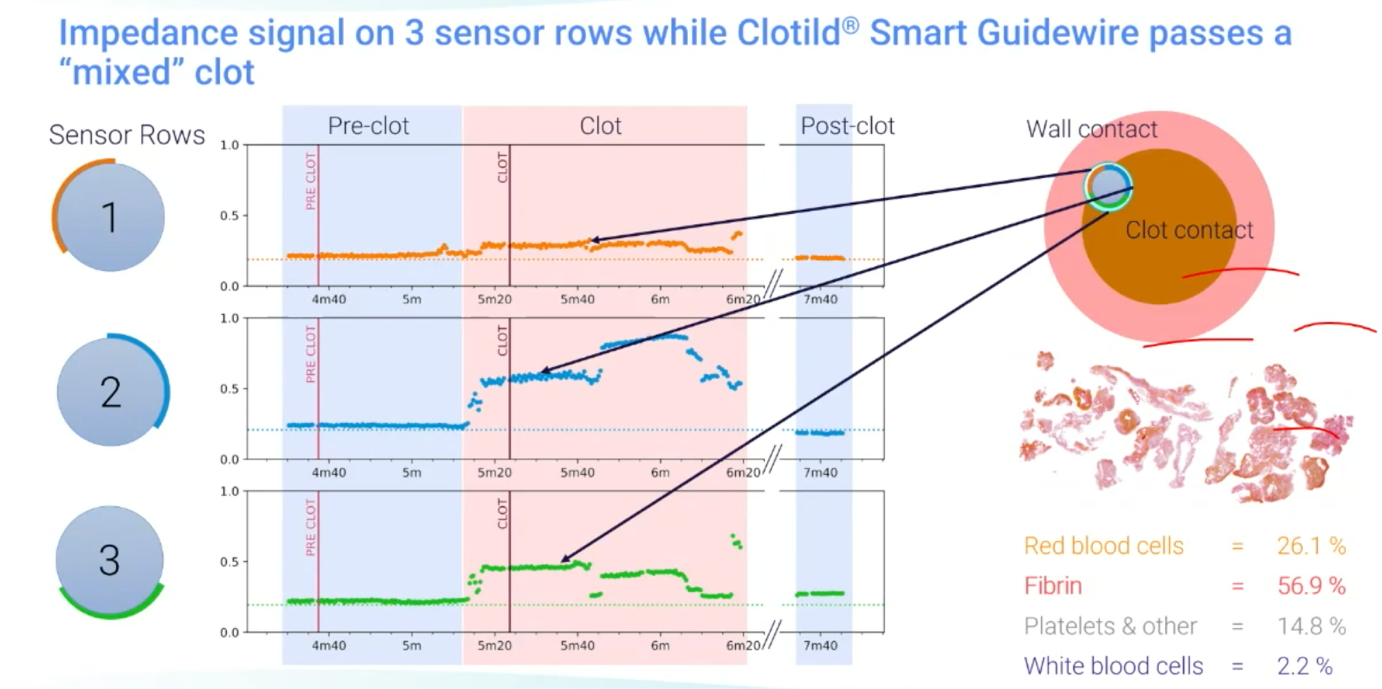
The Clotild is a Smart Guidewire System created by Sensome, which uses artificial intelligence (AI) to provide real-time insights on clot presence and its composition. The guidewire is equipped with a microsensor, which is 2 mm in size and performs impedance measurements that are transmitted to the Cloviz tablet device via a Bluetooth transmitter, providing real-time insight on what the clot looks like, and the length of the occlusion. This translates to the sensor delivering higher impedance measurements in red blood cell-rich clots, and lower impedance measurements for fibrin-rich clots, reporting a good differentiation between the two.
Owing to the nine sensors on the device, arranged into three rows, it is also capable of identifying clots that have a ‘mixed’ composition—which may have substantial amounts of red blood cells, fibrin and platelets in their makeup—with this being signified by different impedance readings between the different groups of sensors.
The Clotild has already shown promising results in the CLOT OUT trial, which is the first in-human study evaluating the safety of the Clotild smart guidewire system. It is the single-arm, multicentre study began in late 2021 which is taking place across Australia and Europe, and intends to enroll roughly 100 patients with an M1-origin acute ischemic stroke in order to collect a total of 60 clots. So far the trial has enrolled 23 patients to date, with no device-related complications being observed in these completed cases, and the first Data Safety Monitoring Board meeting recently recommended continuation of the study.
Future applications of Clotild will go beyond clot composition and length, such as in vessel-wall analysis to elucidate other key occlusion characteristic like smooth muscle content and cholesterol—possibly identifying clots that are actually intracranial atherosclerosis (ICAS) lesions, not thromboembolic occlusions.
Conclusion:
The Clotild provides real-time insights on clot presence and its composition. Its use may be applicable in particular to:
- Stenosis or residual thrombus differentiation
- Prediction of the length of the thrombus
- Identification of branch involvement in case of bifurcation thrombus
- Understanding of clot composition (white vs red clot)
Literature:
- https://neuronewsinternational.com/ai-powered-device-delivers-amazing-physiologic-information-to-guide-thrombectomy-treatments/
- https://evtoday.com/news/sensomes-clotild-smart-guidewire-system-studied-in-clot-out-fih-trial-for-large-vessel-acute-ischemic-stroke
- https://www.sensome.com/